
Recently, Risk Warning, Prevention and Control of Bacterial Infectious Diseases in Livestock and Poultry Team of SHVRI revealed the mechanism on the transfer of mcr-1 , bla CTX-M-14, and bla TEM-176 antimicrobial resistance genes from avian pathogenic E. coli (APEC). This study helped researchers to further understand that APEC was a reservior and involved in the dissemination of antimicrobial resistance genes. The related results were published in Transboundary and Emerging Diseases .
Background
Avian pathogenic Escherichia coli (APEC) is a kind of extra-intestinal pathogenic E. coli (ExPEC), which can cause avian colibacillosis and lead to worldwide economic losses in the poultry industry. The antimicrobials were used to treat APEC infection, however, the inappropriate use of antibiotics accelerated the emergence of multi-drug resistant (MDR) APEC. This not only attenuates the healing effects of antibiotics, but also raises the possibility that drug resistance genes would be transmitted from APEC to human-derived pathogenic bacteria, which poses potential risks to public health.
Research Progress
In order to further reveal the mechanism of antibiotics resistance genes’ dissemination among APEC strains, 200 APEC strains were isolated and identified from diseased avians in China. Among them, EC012 strain displayed resistance of almost all tested antibiotics including beta-lactams, aminoglycosides, quinolones, sulfonamides, phenicol, tetracycline, and colistin. Whole genome analysis showed that EC012 has 6 plasmids. The Conjugation experiments showed two of six plasmids named pEC012-1 and pEC012-5 could transfer into the recipient E. coli 600. Plasmid pEC012-1 co-harbored bla CTX-M-14 and mcr-1 genes, and mediated resistance to beta-lactams and colistin, respectively. The plasmid pEC012-5 harbored bla TEM176 gene, which mediated beta-lactams resistance. The phylogenetic tree built with core genes revealed that EC012 clustered with several avian and human pathogenic E . coli strains. It further stresses out the possibility that avian flocks are becoming the potential reservoir of APEC and disseminating multiple drug resistance genes through transitive plasmids, in the context of widely misuses of antibiotics.
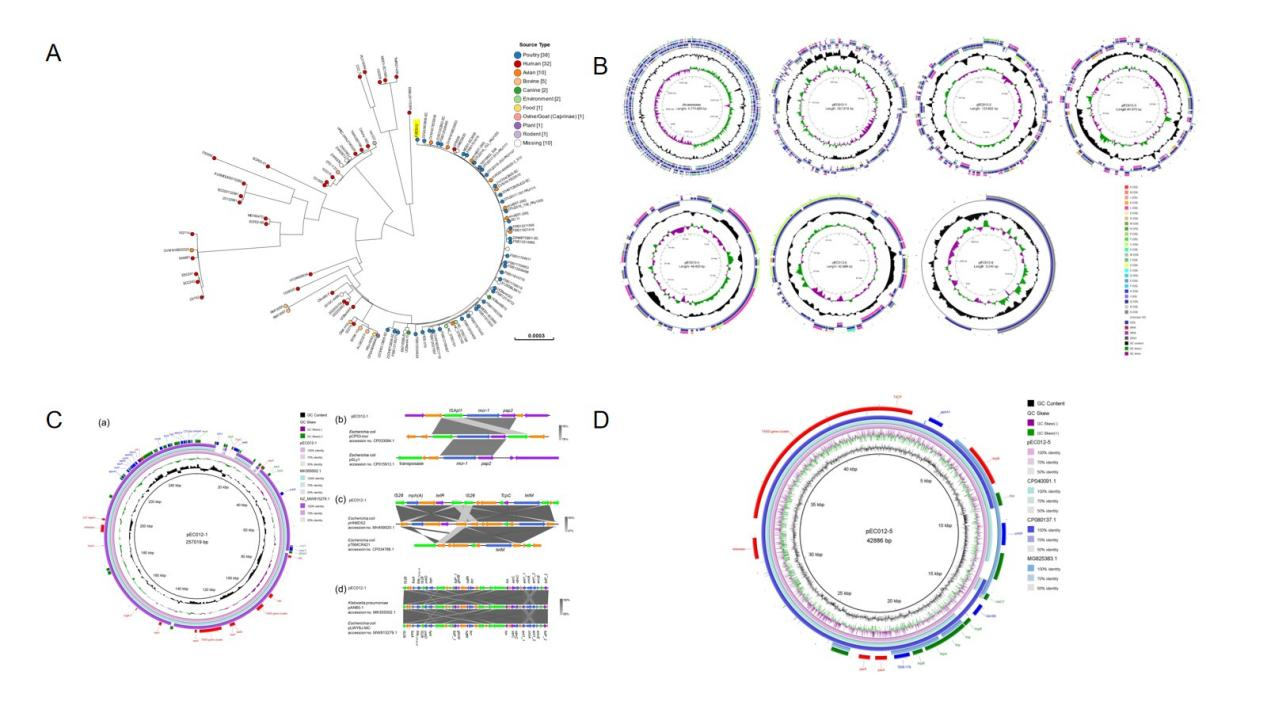
Building of the phylogenetic tree with core genes of EC012 and other strains, the structure of EC012’s whole genome, and comparative genomic analysis of EC012 plasmids
Funding
This work was sponsored by the National Key Research and Development Program of China, National Natural Science Foundation of China, the Natural Science Foundation of Shanghai, and Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences. The master candidate Zhiyang Wang from SHVRI is the first author. Prof. Shaohui Wang, Cheif Scientist of Risk Warning, Prevention and Control of Bacterial Infectious Diseases in Livestock and Poultry Team and Assoc. Prof. Haihua Li from Tianjin Agricultural University were co-corresponding authors.
(Link to original article: https://doi.org/10.1155/2024/9332418)

