
Recently, Risk Warning, Prevention and Control of Bacterial Infectious Diseases in Livestock and Poultry Team of SHVRI summarized the genetic distribution, characterization and function of Escherichia coli type III secretion system 2 (ETT2). It concluded that an intact ETT2 may retain the capacity to translocate effector proteins and manipulate the Escherichia coli (E. coli) pathogenesis and host's innate immune response, suggesting that ETT2 plays an important role during bacterial infections. Further investigations into the structure, function and regulation of ETT2 are imperative for comprehensive understanding of E. coli pathogenicity and the development of effective control strategies. The related review was published in iScience.
Background
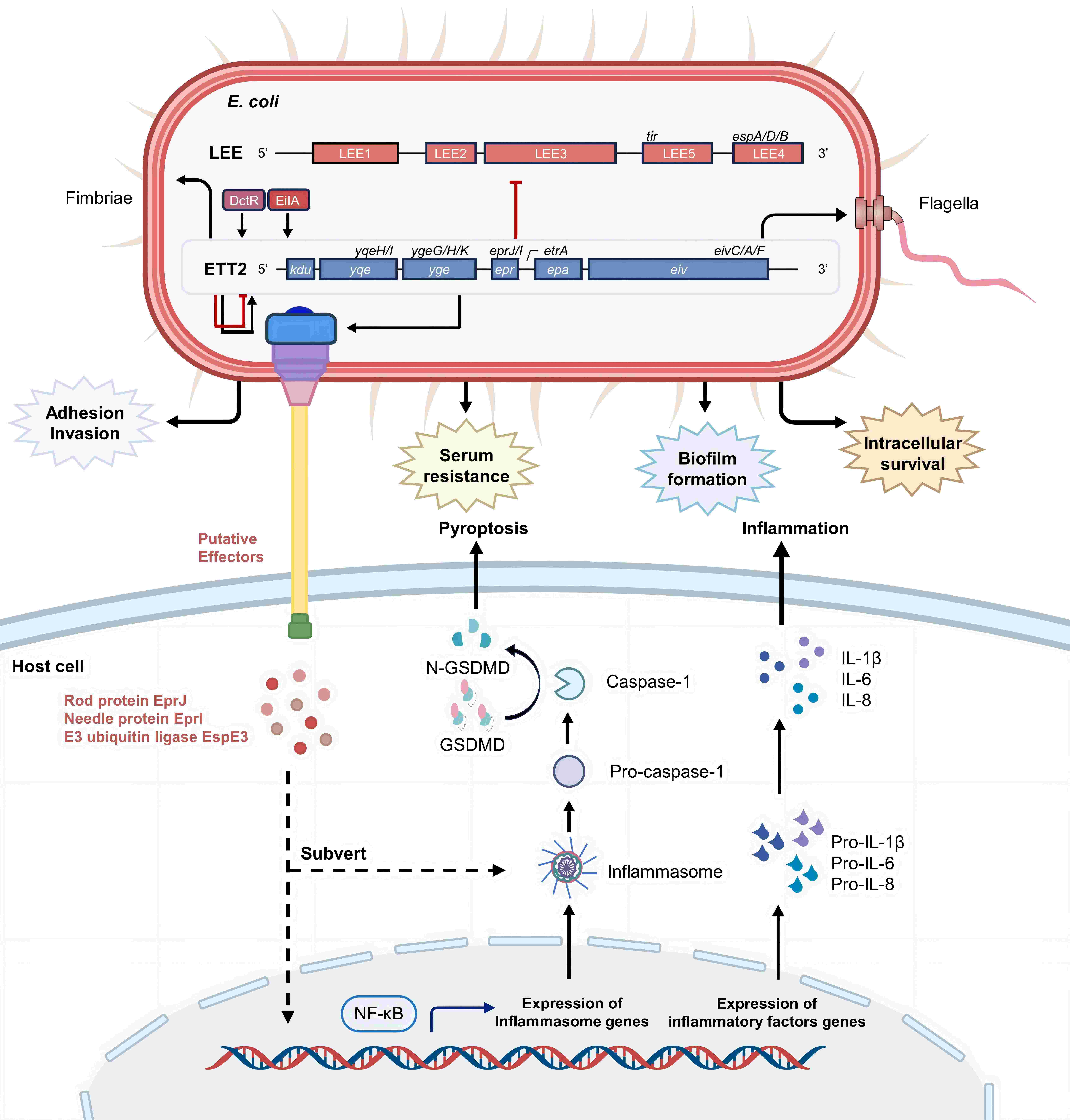
Gram-negative bacteria use type Ⅲ secretion system (T3SS) to inject effector proteins and subvert host signaling pathways, facilitating the growth, survival, and virulence. Some pathogens harbor more than one T3SS, which function independently in different pathogenic processes. Besides a LEE-encoded T3SS, the ETT2 gene cluster was identified in several E. coli . Since many ETT2 carry genetic mutations or deletions, it is thought to be nonfunctional. However, increasing studies highlight ETT2 contributes to E. coli pathogenesis.
Research Progress
ETT2 is widely distributed as intact or deletion mutant in many pathogenic E. coli and E. albertii. Notably, several transcriptional regulators located in the ETT2 virulence gene cluster have been shown to alter adhesion, motility, biofilm formation, serum bactericidal resistance, and intracellular survival of bacteria. Intact ETT2 has the potential to function by secreting potential effector proteins and helping the bacteria to subvert host cellular processes and pathways during host infection. The regulators within the ETT2 gene cluster also contribute to the E. coli pathogenicity by affecting various virulence gene expression. Moreover,ETT2-positive E. coli can also act as a reservoir of ETT2 virulence island transfers, give more strains the opportunity to acquire horizontally transferred genes, thus contributing to the formation of new and complex pathotypes and increasing the risk of disease. However, our current understanding of the ETT2 virulence island is limited. Further research will not only enhance our understanding of bacterial pathogenicity but also aid in identifying new targets for vaccine development.
Funding
This work was sponsored by the National Key Research and Development Program of China, National Natural Science Foundation of China, the Natural Science Foundation of Shanghai, and Agricultural Science and Technology Innovation Program of the CAAS. PhD student Xinyu Wang from SHVRI is the first author, Prof. Shaohui Wang from SHVRI, CAAS and associate researcher Prof. Fusheng Si from Shanghai Academy of Agricultural Sciences were the co-corresponding authors.
Link to original article
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11068852/

