
Recently, Ying Liao's team of SHVRI, CAAS published a study in Plos Pathogens titled “Coronavirus Nucleocapsid Protein Enhances the Binding of p-PKCα to RACK1: Implications for Inhibition of Nucleocytoplasmic Trafficking and Suppression of the Innate Immune Response.” This study is the first to reveal a novel mechanism by which the coronavirus nucleocapsid (N) protein suppresses the expression of antiviral genes by interfering with the host nucleocytoplasmic transport system and inhibiting the nuclear translocation of transcription factors critical to innate immune signaling pathways. This discovery provides significant theoretical support for the prevention and control of coronaviruses.

Background
The nuclear pore complex (NPC) serves as a critical channel for material exchange between the nucleus and cytoplasm, mediating the transport of proteins and RNA. It plays essential roles in gene transcription, RNA export, protein translation, and antiviral immune responses. The NPC is vital for the nuclear translocation of key transcription factors in innate immune signaling pathways, such as IRF3, STAT1, STAT2, and NF-κB, which are crucial for inducing the expression of antiviral genes.
Research Progress
This study found that coronavirus infection causes the disassembly of nucleoporins, disrupting the function of the nucleocytoplasmic transport system and inhibiting the nuclear translocation of transcription factors. Further investigations revealed that the coronavirus-encoded N protein interferes with the host's nucleocytoplasmic transport system through the following mechanisms:
1. Enhancing the interaction between phosphorylated PKCα (p-PKCα) and RACK1, thereby activating PKCα.
2. Inducing p-PKCα-mediated phosphorylation of NUP62, a critical component of the NPC, leading to the disassembly and redistribution of NUP62 and other key nucleoporins to the cytoplasm, thereby weakening nucleocytoplasmic transport capacity.
3. Preventing the nuclear translocation of multiple transcription factors, thereby blocking the expression of antiviral and pro-inflammatory genes.
This study systematically reveals a novel mechanism by which coronaviruses evade innate immunity by disrupting the host NPC function. This finding is not only a novel discovery in virology but also a breakthrough in cell biology: identifying PKC as a new kinase for NUP62 and confirming that phosphorylation of nucleoporins forms the basis for nuclear pore disassembly. It also identifies potential targets for the development of antiviral drugs. These findings deepen our understanding of the interactions between viruses and the host immune system and provide valuable insights for controlling coronaviruses.
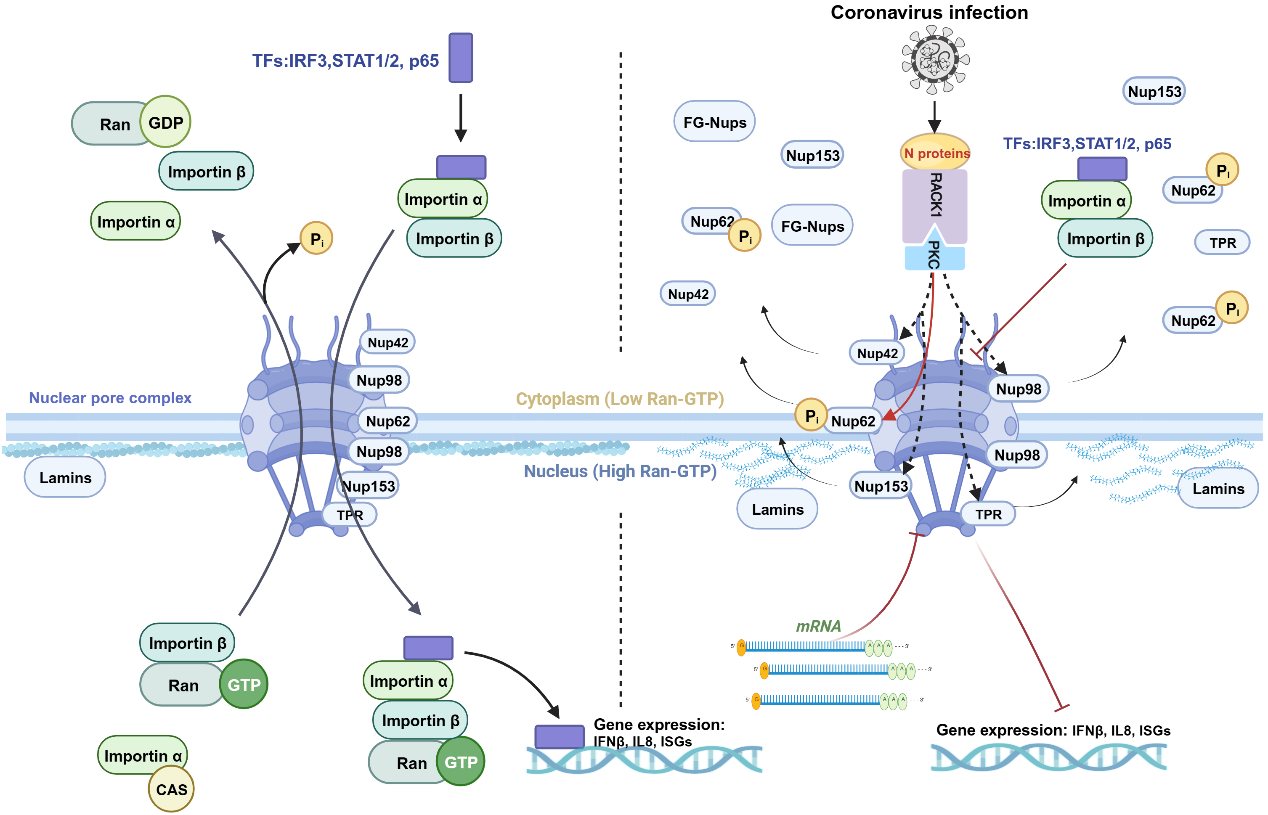
Coronavirus N protein regulates the nucleocytoplasmic transport system to antagonize host immune responses
Funding
Prof Ying Liao from the Shanghai Veterinary Research Institute of CAAS is the corresponding author of the paper, and Ph.D. student Wenxiang Xue is the first author. This study was supported by funding from the National Key R&D Program of China (2021YFD1801104), the National Natural Science Foundation of China (32372999 and 32172834), and the Shanghai Natural Science Foundation (23ZR1477000).
Link to Original Article
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1012097

Left: Dr. Wenxiang Xue, first author; Middle: Prof. Ying Liao, corresponding author; Right: Mr Jiehuang Wang, co-author (third)

