
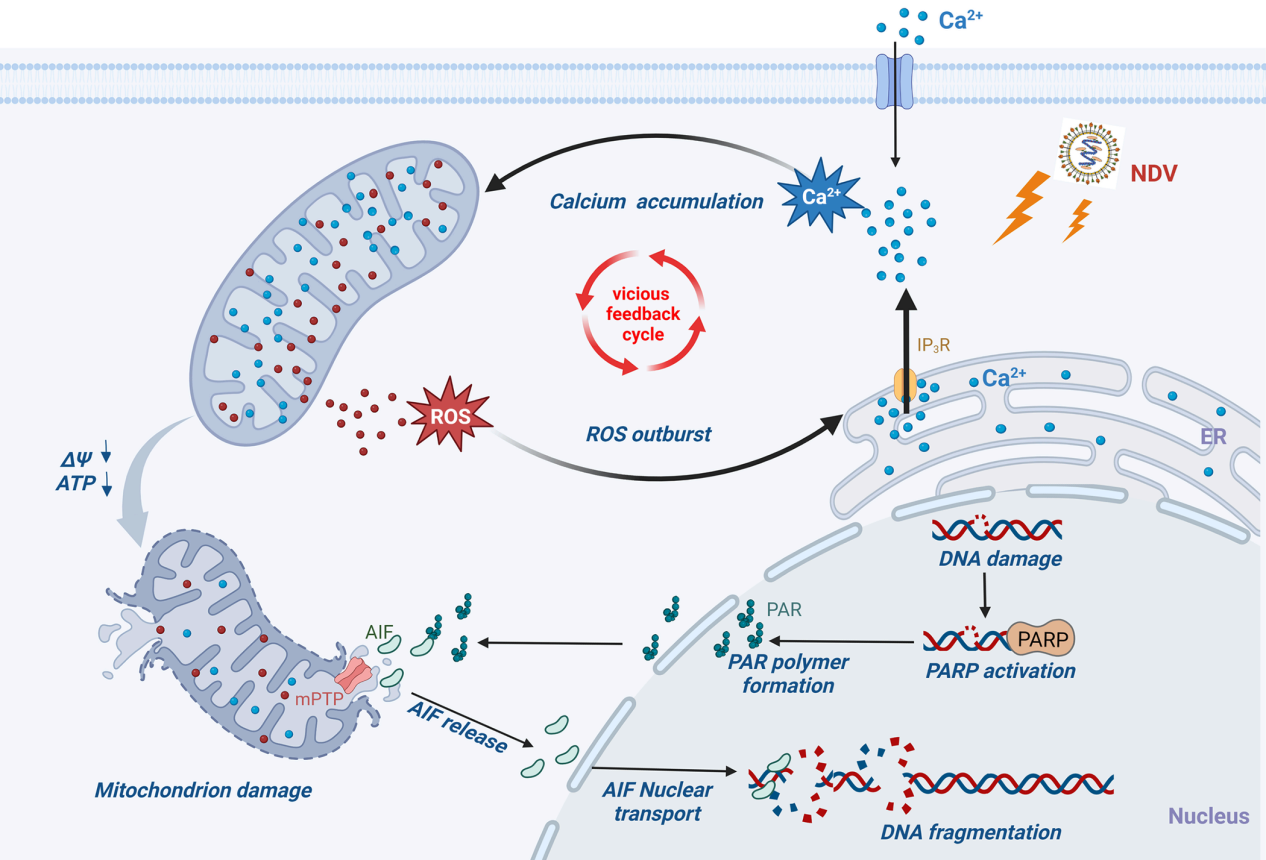
The Poultry Viral Disease Team of SHVRI, CAAS recently uncovered a novel mechanism by which Newcastle disease virus (NDV) induces mitochondrial oxidative stress and functional damage. Their study revealed that NDV infection increases intracellular free calcium ions (Ca²⁺), triggering the opening of mitochondrial membrane permeability transition pores (mPTP). This leads to the release of mitochondrial apoptosis-inducing factor (AIF) into the nucleus, ultimately activating caspase-independent Parthanatos cell death. These findings were published in PLOS Pathogens.
Background
Apoptosis, a form of programmed cell death, is tightly regulated by complex signaling pathways and functions to eliminate unwanted or harmful cells. It can be initiated via the extrinsic pathway mediated by cell death receptors or the intrinsic pathway linked to mitochondrial dysfunction. Both pathways rely on caspase family proteolytic enzymes. In contrast, Parthanatos is a distinct type of cell death that occurs independently of caspases. It involves the nuclear translocation of AIF, resulting in extensive DNA fragmentation (20-50 kb). Key features of Parthanatos include the overactivation of PARP1 and the accumulation of PAR polymers.
Research Progress
This study demonstrated that NDV infection leads to a pathological buildup of Ca²⁺ in mitochondria, primarily due to the acute release of calcium waves from the endoplasmic reticulum, the cell's main calcium reservoir. Excessive mitochondrial calcium accumulation results in oxidative stress, structural damage, and functional impairment, promoting AIF release from mitochondria. Once translocated into the nucleus, AIF triggers extensive DNA fragmentation and cell death. Furthermore, the study highlights the interplay between calcium signaling and reactive oxygen species (ROS), a byproduct of oxidative stress, in NDV-induced cell death. Elevated intracellular Ca²⁺ levels contribute to NDV-induced ROS accumulation, while the elimination of ROS significantly reduces the NDV-induced rise in cytoplasmic and mitochondrial Ca²⁺ levels. In summary, this research elucidates how Ca²⁺ and ROS mediate NDV-induced, AIF-dependent Parthanatos through the Ca²⁺-mitochondria-ROS signaling axis. These findings provide novel insights into the mechanisms by which NDV disrupts cellular processes, offering valuable knowledge and a foundation for future antiviral research and therapeutic strategies.
Funding
Dr. Yang Qu, a postdoctoral fellow at the Shanghai Veterinary Research Institute, is the paper's first author. Professors Chan Ding and Yingjie Sun from the Shanghai Veterinary Research Institute, along with Professor Zengqi Yang from Northwest A&F University, serve as co-corresponding authors. This research was supported by grants from the National Natural Science Foundation of China and the National Key R&D Program.
Link to Original Article
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1012737

