
Recently, the Animal Parasitology and Vector Control Group of SHVRI, CAAS, demonstrated that Babesia microti infection promotes ferroptosis in ticks through downregulation of the histamine-releasing factor (HRF) pathway. These findings were published in Frontiers in Cellular and Infection Microbiology.
Background
Ticks serve as vectors for a diverse range of pathogens, including Borrelia burgdorferi, Rickettsia rickettsii, tick-borne encephalitis virus, and Babesia spp., posing significant threats to human health. HRF (Histamine-Releasing Factor) molecules in ticks are highly conserved eukaryotic proteins involved in critical biological processes such as apoptosis and autophagy, which protect cells from oxidative stress damage. Ferroptosis, an iron-dependent form of regulated cell death, is characterized by the accumulation of intracellular lipid peroxides, ultimately leading to membrane rupture and cell death. Emerging evidence suggests that modulating the ferroptosis pathway may inhibit pathogen infection. However, the relationship between ferroptosis and B. microti. infection in ticks remains poorly understood, warranting further investigation.
Research progress
In the present study, using a B. microti-tick-mouse infection model, we demonstrated that B. microti induces ferroptosis in engorged ticks at the engorged 0 day. This was characterized by distinct ultrastructural changes in the midgut, including reduced mitochondrial cristae, smaller mitochondria, and a significant accumulation of ferrous iron (Fe²⁺) and reactive oxygen species (ROS). Consistent with ferroptosis, qPCR and Western blot analyses revealed the downregulation of Ferritin 1 and GPX4. Next, we use of ferroptosis promoter Erastin could promote Babesia infection, and inhibitor Ferrostatin-1 could inhibit Babesia infection.
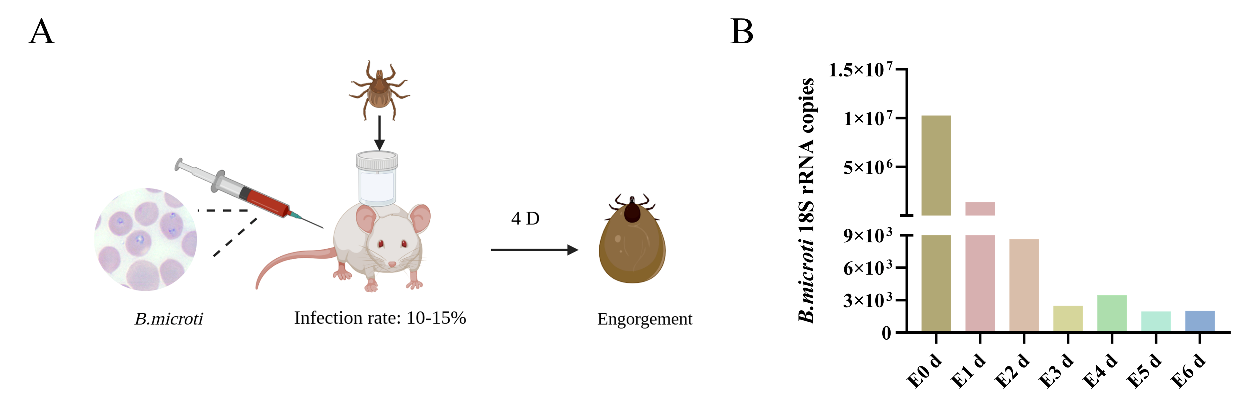
B. microti presence in the engorged nymph and detection
Further studies revealed that B. microti infection caused down-regulation of tick HRF molecules. Transcriptomics analyses showed that interfering with HRF expression promoted ticks to undergo iron death, mainly through the down-regulation of Ferritin1 and GPX4. Compared with controls, ticks with interfered HRF had increased midgut divalent iron ions, ROS, and reduced mitochondrial ridges. This study reveals the importance of tick HRF molecules in the regulation of ferroptosis and B. microti infection, providing new insights for a deeper understanding of tick-B. microti interactions. Our study highlights the critical role of tick HRF in regulating ferroptosis and Babesia infection, providing novel insights into the molecular interactions between ticks and B. microti.
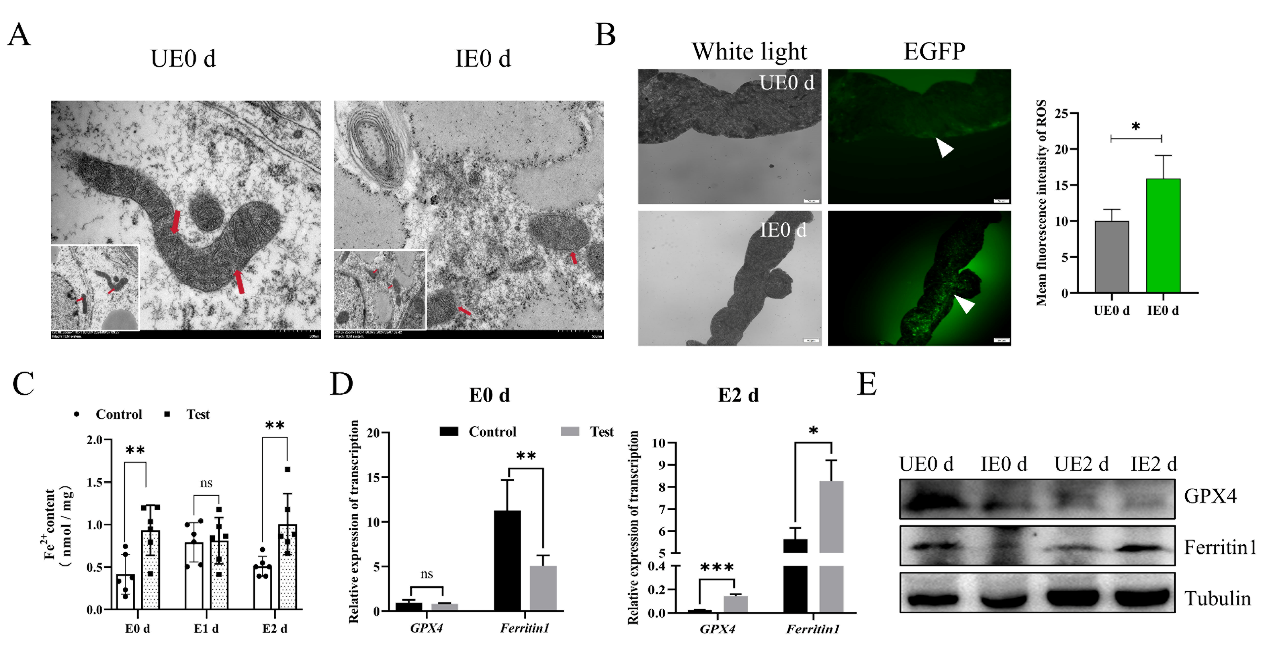
B. microti promotes tick ferroptosis
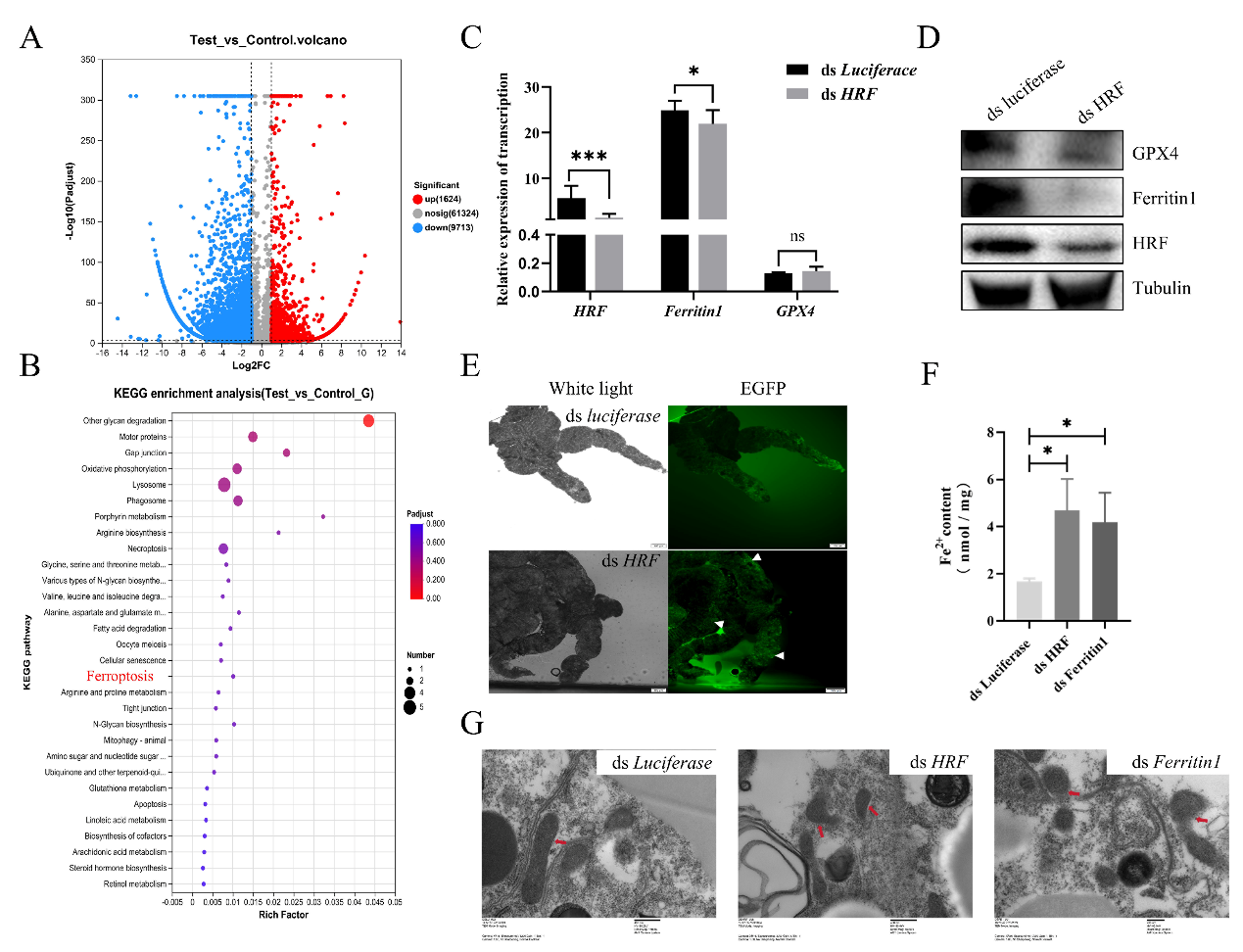
Tick HRF molecules promote ferroptosis
Funding
Chen Songqin, a PhD of SHVRI, CAAS, served as the first author of this study, Professor Zhou Jinlin was the corresponding author. This work was supported by the National Key Research and Development Program of China.

