
Recently, the Companion Animal Biological Safety Risk Warning and Control Technology team of SHVRI, CAAS,successfully developed a novel cat herpesvirus (FHV-1) and feline calicivirus (FCV) bivalent live vector vaccine, based on a weakened strain of FHV-1. Safety and efficacy trials have shown that the live vector vaccine is safe, effective, and of controllable quality, providing strong protection against both FHV-1 and FCV infections. The research results have been published in Vaccine .
Background
FHV-1 and FCV are major pathogens that cause upper respiratory diseases in cats. Existing inactivated or live vaccines for FCV and FHV have certain limitations in terms of safety and efficacy. To address these challenges, the research team utilized a previously constructed weakened FHV-1 strain (Virol J. 2023; 20(1):87) as the backbone and employed CRISPR/Cas9-mediated homologous recombination to insert the antigen gene of the highly pathogenic FCV (VSD-FCV) into the gI/gE region of the FHV-1 genome. This led to the successful development of the FHV-FCV bivalent live vector vaccine.
Research Progress
The team constructed a recombinant FHV virus, FHV ΔgI/gE-FCV VP1, which expresses the FCV capsid protein. The study demonstrated that when CRFK cells were infected with the recombinant virus, it efficiently expressed the VP1 protein and assembled into FCV-like particles (VLPs). Challenge protection experiments confirmed that immunization with the recombinant virus effectively stimulated the production of antibodies against FCV VP1 protein three weeks after immunization, providing protection against VSD-FCV infection. These findings confirm the efficacy of the FHV-based recombinant vaccine in preventing FCV infection and lay the foundation for the development of future multi-valent vector vaccines. This research not only demonstrates the efficacy of an FHV-1-based recombinant vaccine in preventing FCV infection but also provides a critical theoretical and technical foundation for the development of multi-valent vector vaccines. The study’s results will contribute to improving the safety and efficacy of pet vaccines and offer new solutions for pet health management.
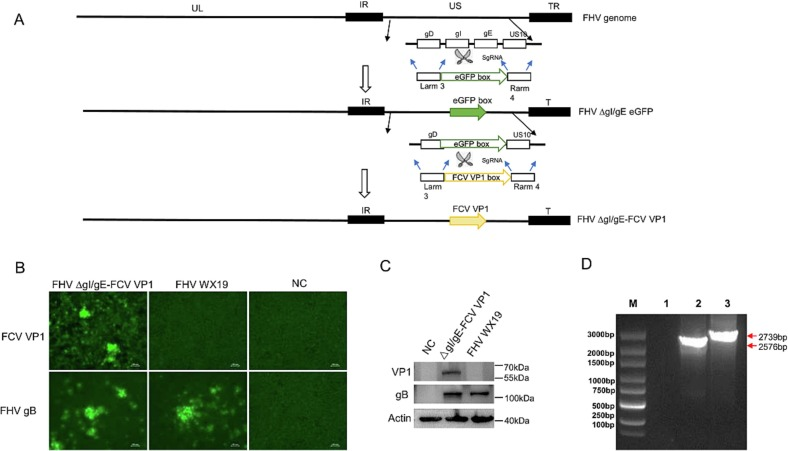
Construction of FHV-FCV Recombinant Virus
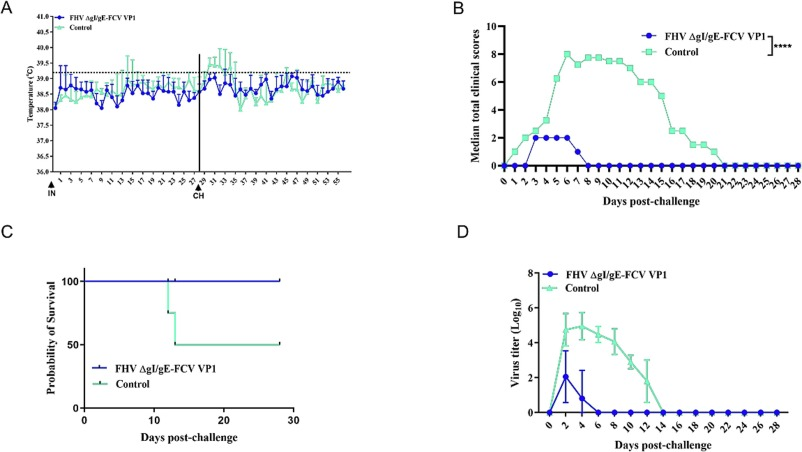
Evaluation of the Immunogenic Efficacy of the FHV-FCV Recombinant Live Vector Vaccine
Funding
This study was supported by the Shanghai Natural Science Foundation (22ZR1476300, 23ZR1477100), Shanghai Science and Technology Major Projects (ZD2021CY001), the 14th Five-Year Plan for Key Research and Development Programs on Zoonotic Virus Infections and Pathogenesis (2022YFD1800100), and the National Natural Science Foundation of China (32172832, 32272982). The first author of the paper is Dr. Tang Aoxing, and the corresponding author is Professor Liu Guangqing.
Link to Original Article
https://pubmed.ncbi.nlm.nih.gov/39467408

