On August 23rd, with the releasing of Announcement No. 701 by the Ministry of Agriculture and Rural Affairs of China, the Inactivated Feline Core Vaccine for feline rhinotracheitis, feline calicivirus disease, and feline panleukopenia (WX strain+SH14 strain+0918 strain) developed by SHVRI CAAS has passed the emergency evaluation and obtained production approval. This marks the breakthrough for feline core vaccine in China, breaks the monopoly situation of long-standing dependence on import, and has the significance impact for promoting the healthy development of China's pet cat industry.
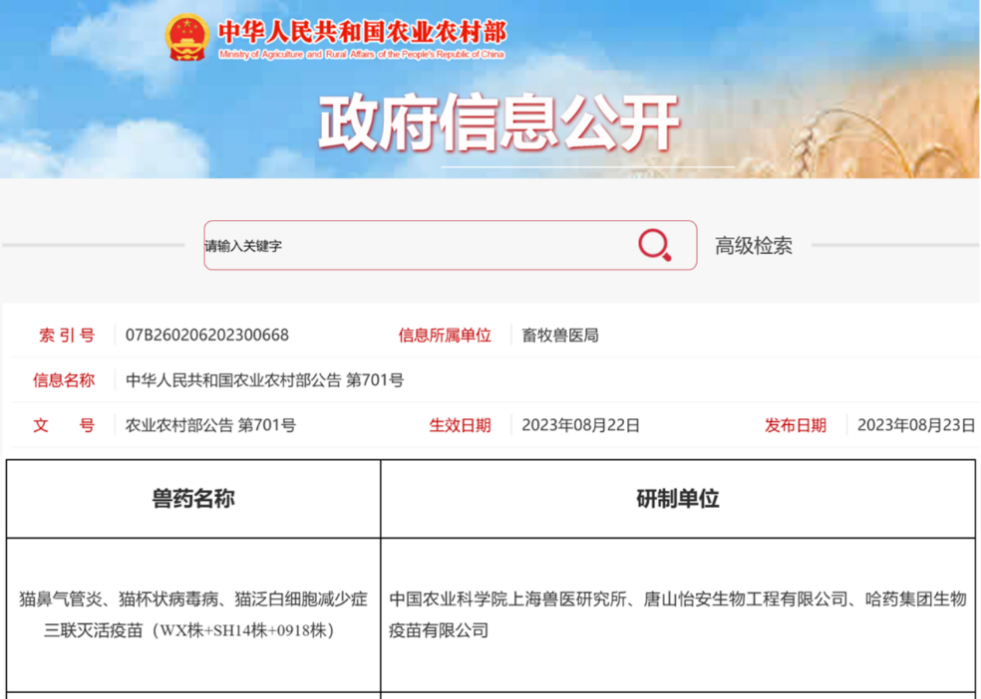
▲ Announcement No. 701 by the Ministry of Agriculture and Rural Affairs
Recent years, with the improvement of people's living standard, the breeding of pet cats has been accelerating. Statistics show that by the end of 2022, the number of pet cats in China was at least 65 million, making it the largest pet population in China. Feline rhinotracheitis, feline calicivirus disease, and feline panleukopenia are the top three common infectious diseases of cats. For a long period of time, vaccines that prevent upper three infectious diseases have fully relied on imports, furthermore with limited amount and high price. The Biosafety Risk Warning and Prevention and Control Technology for Companion Animals team of SHVRI CAAS aimed the actual needs of our nation and society, conducted research and development work on vaccines targeting the upper three core infectious diseases. All team members have sharpened their swords for ten years and ultimately screened the dominant epidemic strains from a large number of clinical samples, based on which developed the Inactivated Feline Core Vaccine.

▲ Patent Certificate for Supporting Testing Methods
According to Prof. Liu Guangqing, leader of feline core vaccine R&D team, this Inactivated Feline Core Vaccine provides more protection for the health and safety of pet cats with its advantages such as high security, fast antibody production, long immune duration, and one shot for three diseases.
During the development of felina core vaccine, the research team also independently developed a matching antigen and antibody detection method, overcomed the dilemma of no experimental animals standardization for cats in China, which has been granted a national invention patent. Associate Prof. Meng Chunchun, the vaccine creator, stated that these testing methods have laid a solid foundation for the establishment of relevant standards for experimental animals in China and the series biological products development for cats.

